High Energy Density Reserve Lithium-Oxygen Batteries with Internal Chemical Oxygen Generation
Single-use reserve batteries for military applications range from ordinance guidance systems to torpedoes, smart ammunition, mines, sonobuoys, unattended ground sensors, UAVs, artillery fuses, and many others. These types of batteries must be able to operate in the harshest outdoor environments over a wide temperature range (-55°C to 125°C); they must be ready on very short notice with activation times of less than 100 ms; and must have extremely long shelf lives (>20 years). Thermal batteries and liquid reserve batteries have generally filled these requirements. While the military systems are becoming more sophisticated and miniaturized, the reserve battery technologies used to power them have remained essentially unchanged for decades. Traditional primary liquid reserve batteries rely on the lithium thionyl chloride (Li-SOCl2) chemistry. Although these batteries have an impressive theoretical energy density, high operating voltage, and good discharge rate capability, practical reserve configurations can only deliver a small fraction of the theoretical storage capacity. This is caused by the need of storing the toxic and corrosive thionyl chloride liquid electrolyte, separately from the rest of the battery, inside a bulky liquid ampoule that needs to be ruptured during the activation step.
Lithium-oxygen batteries (LOBs) have the highest theoretical energy density of all lithium metal batteries. In their simplest configuration, LOBs consist of a highly porous cathode electrode, a Li metal anode, an ionic conducting separator, and an organic electrolyte. During the discharge step, Li metal combines with oxygen to form insoluble lithium peroxide that accumulates in the porous cathode electrode structure. Despite the considerable advantages, LOBs also face several challenges, such as low practical energy efficiency, short cycle life, and poor rate capacity, that have hindered their widespread use and commercialization.


Wasatch Ionics LLC, in collaboration with Brigham Young University, are working on the development of next generation reserve batteries, based on high energy density lithium-oxygen chemistry with integrated chemical oxygen generation (COG). Our novel high surface area/high mesopore volume fraction oxygen cathode electrode maximizes battery discharge capacity by providing a very large storage capacity for lithium peroxide, while enhancing oxygen diffusion and liquid electrolyte wetting. The use of high pure oxygen enables the fabrication of a self-contained and hermetically sealed battery design, therefore, eliminating the adverse effects of moisture, nitrogen, and carbon dioxide, which are present in batteries, where oxygen is sourced from air. The result of this innovation is a reserve battery that delivers a significant improvement in energy density and ultra fast activation, as compared to all of today’s commercial lithium thionyl chloride liquid reserve batteries. Two US patents have been filed on our reserve LOB technology.
Environmentally Friendly Lithium-Ion Battery Recycling Technology
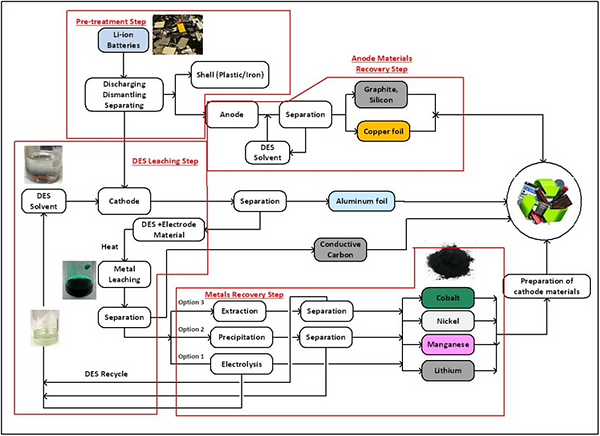
Recycling of spent lithium-ion batteries (LIBs) has become a necessity to remediate the economic and environmental impacts caused by the tremendous growth of this technology. High energy density LIBs contain large quantities of high-value metals such as cobalt, nickel, and manganese, for which there is limited domestic U.S. supply. At present, recycling is mainly limited to portable batteries since there are no significant volumes of waste electric vehicle batteries that have reached their end-of-life. However, it is expected that by 2030, 11 million tons of LIBs from electric vehicles will

reach their end of service life. Therefore, the recovery of high-value metals from spent LIBs offers a great opportunity to establish a viable competitive and domestic supply chain. Traditional pyrometallurgical LIB recycling methods, that rely on high temperature smelting processes, are very energy-intensive and can generate emissions of toxic gases that need to be mitigated. Alternatively, low-temperature hydrometallurgical processes use chemical leaching to extract the metals from the electrode active components and they generate higher purity materials. However, this treatment involves the use of aqueous solutions containing strong inorganic acids and reducing chemicals to dissolve the metals, which are hazardous to humans and the environment.
Wasatch Ionics is working in the development of an environmentally friendly hydrometallurgical process alternative that uses a new type of “green solvents”, called Deep Eutectic Solvents (DESs). DESs have the ability of extracting and reducing transitional metal oxides in a single processing step at low temperatures. These solvents can be easily synthesized from non-toxic, biodegradable, and inexpensive raw materials. In addition, DESs are liquid electrolytes with wide electrochemical windows, which enable the selective recovery of the metals by electrodeposition. Wasatch Ionics has demonstrated that selected DES solvents can reach up to 99% cobalt and nickel extraction efficiencies from multiple cathode LIB materials such as NMC111, NMC 811, and LCO.
Wasatch Ionics full hydrometallurgical lithium-ion battery recycling scheme enabled by Deep Eutectic Solvents
Our company has partnered with a computational chemistry group, led by Dr. Johannes Hachmann at the University at Buffalo-SUNY, to design and screen out the most promising DES candidates for this LIBs recycling application.
Wasatch Ionics has received a U.S. Department of Energy Phase I SBIR grant to advance this technology to the next stage of development.
Novel Miniature Reserve Batteries on the Chip
The U.S. Army has an increasing need for microscale reserve battery systems that can be integrated into electronic devices, sensors, and other power-consuming components of munitions. These micro-battery systems must be able to operate in the harshest outdoor environments over a wide temperature range, be ready on very short notice with fast activation times, and must have extremely long shelf lives.
In collaboration with the U.S. Army, Wasatch Ionics is working towards the development of a scalable reserve micro-battery architecture using Direct Ink Writing (DIW) and other advanced additive manufacturing techniques. DIW can fabricate complex 3D objects via digitally controlled deposition of solvent-based inks directly onto a substrate with microscale precision. A significant advantage of this 3D printing approach is that it enables the implementation of very different types of primary and rechargeable battery chemistries, simply by modifying the composition of the active components of the inks. The reserve microbattery device incorporates a proprietary superhydrophobic structured membrane that keeps the battery in the inactive state by separating the electrolyte from the electrodes by capillary forces. Quick battery activation can be accomplished by inertially or electrically changing the wetting state of the electrolyte on top of the membrane, and therefore allowing the liquid electrolyte to move through the separator to come in contact with the battery active electrodes. This architecture is highly compatible with the implementation of power management features, where individual batteries or groups of them can be activated on demand and be arranged in different electrical configurations.
Fabrication of micro-battery by Direct Ink Writing
Reserve micro-battery grid configuration concept developed by Wasatch Ionics


Micro-battery prototype fabricated by Direct Ink Writing with electrolyte encapsulation


